Signal Amplification |
| Most of the amplification in the chemotaxis pathway occurs in receptor signaling complexes. How receptor complexes produce high signal gain is still an open question, but work from our laboratory and from a number of other groups over the past ten years has led to an explicit working model in which receptors of different types collaborate in signal teams (see Parkinson, et al., 2005; Hazelbauer, et al., 2008 for reviews of this topic).
Signal gain through clustering The first clue to the amplification mechanism of chemoreceptors was provided in 1993 by Maddock & Shapiro, who discovered that the E. coli receptors resided in large clusters at the cell poles (Fig. 1). MCP-family receptors in many other bacterial species have since been shown to cluster in similar fashion. In 1998, Dennis Bray and colleagues suggested that clustering could enable receptor molecules to generate amplified output signals through physical, information-sharing interactions with one another (Fig. 1). |
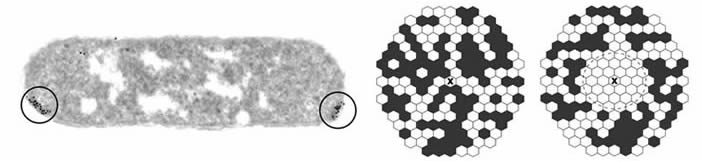 |
| Fig. 1 - Chemoreceptor clusters. Left: Thin-section electron micrograph of clustered receptor molecules in a wild-type E. coli cell. The receptors are labeled with a gold-tagged antibody directed against their conserved signaling domains (Maddock & Shapiro, 1993). Right: Model of signal spread through a receptor cluster. Receptors in the kinase-off signaling state are white; those in the kinase-on state are black. The receptor indicated by X has just bound an attractant ligand, switching it to the off state. Clustering could enable a receptor to amplify ligand-binding signals by imposing its signaling state on its neighbors through conformational spreading (Bray et al., 1998). |
| An important insight into the physical basis for receptor-receptor interactions was provided by Kim et al. in 1999, who reported that a soluble fragment of the Tsr signaling domain crystallized as a trimer of dimers. Until then, it was widely assumed that receptors functioned as homodimers, the native state of the chemoreceptor molecule. The crystal structure of Kim et al. raised the possibility that receptor molecules might function in higher order complexes through trimer-of-dimer associations. Below, we summarize studies from the Parkinson Lab that receptor trimers of dimers, indeed, form in vivo and represent the key architectural element of receptor signaling complexes and macromolecular arrays.
In vivo evidence for receptor trimers of dimers We devised several crosslinking approaches, guided by the Kim, et. al., trimer structure for Tsr, that demonstrated trimer formation by receptors in vivo. One approach (Fig. 2) employed a trifunctional thiol-reactive compound, TMEA, that freely enters cells. The reactive maleimide groups of TMEA are spaced about 10 Å apart, in a trigonal pattern. Receptor molecules that have a cysteine reporter positioned just above the predicted trimer contact region react with TMEA to form crosslinked products that contain two or three receptor subunits. Several lines of evidence indicate that these products arise from the axial subunits in trimers of dimers: (i) About 50% of the reporter subunits are crosslinked, consistent with a lack of close contacts between the outer subunits in trimers (see Fig. 2). (ii) Receptors of different types, which cannot form heterodimers, nevertheless form mixed TMEA crosslinking products in proportions consistent with random association of receptor dimers in trimers (see Fig. 2). [The principal trimer contact residues in all five MCP-family receptors of E. coli (Tsr, Tar, Tap, Trg, Aer) are identical.] (iii) Single amino acid replacements at predicted trimer contact residues can impair or abolish trimer formation. Such lesions abrogate receptor signaling function by preventing ternary complex assembly. |
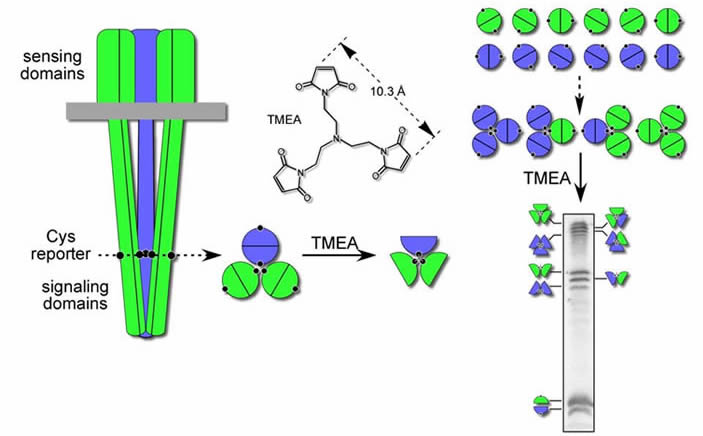 |
| Fig. 2 - In vivo crosslinking assay for receptor trimers of dimers. In this example, two different types of receptors (Tsr = green, Tar = blue), each bearing a unique cysteine replacement just above the predicted trimer contact region, are crosslinked with the trifunctional thiol-reactive agent TMEA. The resulting products can be identified by their distinct electrophoretic mobilities in a denaturing polyacrylamide gel. [Note: Tar and Tsr subunits do not form heterodimers, but do form mixed trimers of dimers.] |
In vivo evidence for collaborative signaling interactions between receptors Some Tsr trimer contact mutations (Tsr*) destroy receptor function, but do not abrogate trimer formation. Many of those mutant receptors exhibit altered signaling behavior that is correlated with, and presumably dependent on, trimer formation. Some of the mutant signaling defects are phenotypically rescued in cells that contain a functional receptor of another type (Fig. 3). Other mutant receptor defects prevent or "jam" the function of heterologous receptors. These sorts of functional interactions between receptors of different detection specificities argue that receptors of different types operate collaboratively in higher-order complexes. |
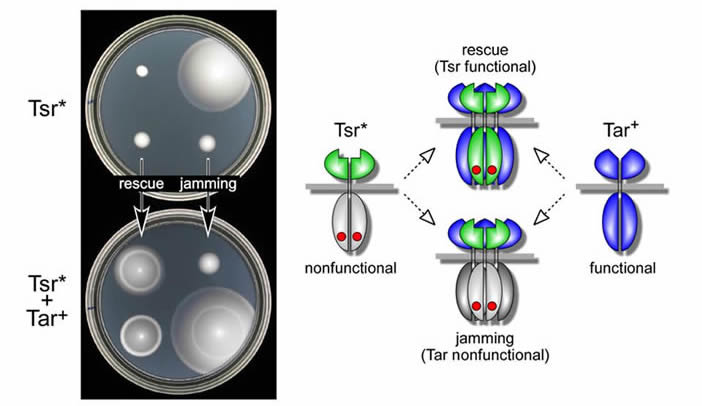 |
| Fig. 3 - Rescuable and jamming receptor defects. Left: Chemotaxis phenotypes conferred by mutant Tsr receptors, either alone (top) or in the presence of wild-type Tar receptors (bottom). One of the Tsr lesions is functionally rescued by Tar, another jams Tar function; a third shows neither effect. Right: Model of functional rescue and jamming. Receptors of different types are proposed to function in higher-order groups ("signal teams") that are probably based on trimer-of-dimer complexes. |
Conformational suppression of inter-receptor signaling defects If mutant receptors jam heterologous receptors through mixed trimer formation, it might be possible to "rescue" a jamming defect by altering the conformation of the heterologous receptor in the mixed trimer. We looked for such suppressor changes in the aspartate receptor, Tar, and found a variety of mutations that could restore signaling function to a mutant Tsr receptor with jamming behavior. (Fig. 4). The suppressor mutations (Tar^) invariably occurred at the tip of the receptor, where the trimer contact sites are located. Some mutations even arose at a trimer contact site in Tar (Fig. 4, yellow residues). The Tar^-Tsr* suppression patterns were highly allele-specific, consistent with restoration of function through compensatory conformational changes. The existence of conformational suppression effects between heterologous receptors argues that receptors of different types function together in a higher-order complex. Although the genetic evidence alone cannot tell us the structure of that complex, the most parsimonious possibility is a trimer of dimers. |
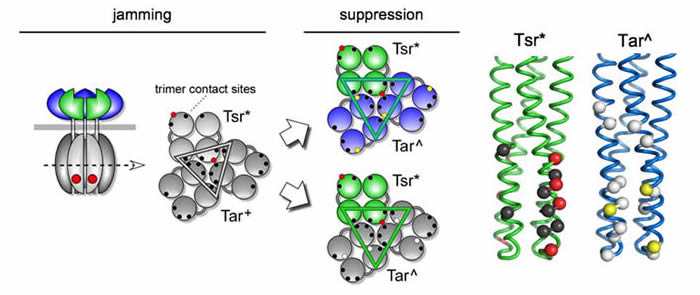 |
| Fig. 4 - Inter-receptor suppression of trimer contact lesions. Mutant receptors (Tsr*) with a jamming lesion at a trimer contact residue (red) are proposed to distort the geometry and operation of trimer-based receptor groups; gray shading indicates lack of signaling function. The jammed receptor (Tar+) can acquire single-step mutations (Tar^) that functionally rescue the jamming receptor, restoring serine chemotaxis ability. Some Tar^ suppressors allow function by both receptor types (top), others only allow the jamming Tsr* receptor to function (bottom). The different behaviors of the suppressors presumably reflect the ways in which they influence the structure and function of mixed trimers. Tar^ suppressors only arise in or near the Tar trimer contact residues (yellow and white sites, respectively). They suppress some of the suppressible Tsr* trimer contact defects (red), but no lesions at other contact sites (black). |
The team model of receptor signaling and cluster formation Our current view of the receptor cluster is that it represents a networked array of receptor signaling teams (Fig. 5). The team is the fundamental (smallest) unit of receptor signaling and corresponds to one or several receptor trimers and their CheA/CheW partners. |
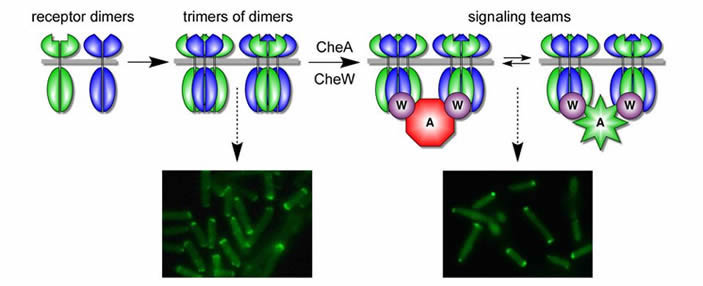 |
| Fig. 5 - Team model of receptor signaling and clustering. Receptor dimers of different types coalesce into trimers of dimers which form extended polar clusters (caps). In the presence of CheW/CheA, trimers form signaling teams that modulate CheA activity in response to chemoeffector stimuli. Teams with shared connections to their CheA and CheW partners form tight polar clusters, representing the networked receptor array. |
We do not yet know the mechanistic or architectural details of receptor arrays. A combination of structure-function approaches-at organismal to molecular levels-will be needed to understand the high-gain signaling properties of E. coli chemoreceptors. |
