An overview of E. coli chemotaxis |
|
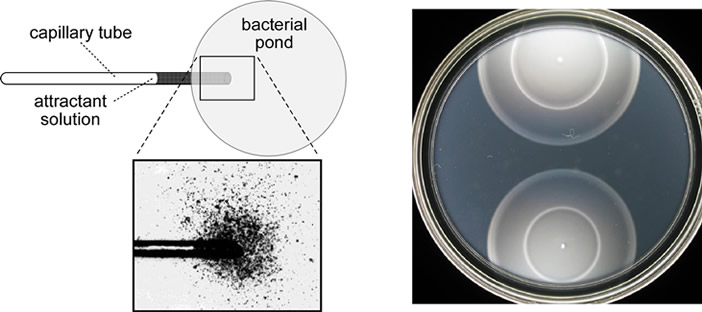
Chemotactic behavior Chemotaxis, movement toward or away from chemicals, is a universal attribute of motile cells and organisms. E. coli cells swim toward amino acids (serine and aspartic acid), sugars (maltose, ribose, galactose, glucose), dipeptides, pyrimidines and electron acceptors (oxygen, nitrate, fumarate). Figure 1 shows two simple methods for assessing attractant responses by E. coli. The capillary assay relies on diffusion-generated gradients and is more quantitative, but also more laborious. Soft agar assays involve metabolism-generated chemoeffector gradients and provide a more qualitative, but expedient, measure of chemotactic ability. E. coli also swims away from potentially noxious chemicals, such as alcohols and fatty acids, but repellent responses haven't been as extensively studied. |
|
 |
|
| Fig. 1 - Two assays of chemotactic behavior in E. coli. Left: A capillary assay invented by W.Pfeffer in the 1880's. The test chemical diffuses from the capillary mouth, establishing a steep gradient that attracts bacteria to the entrance. The cells enter the capillary and are subsequently documented by colony counts. Right: A tryptone soft agar plate in which motile cells can swim through water-filled tunnels in the agar. Two chemotactic colonies are shown after ~8 hours incubation at 30°C. As the cells grow, they establish attractant gradients through consumption of carbon and energy sources. This medium contains two attractants, serine, which the cells eat first, and aspartate, which they eat when serine is unavailable. These strains detect and respond to both chemicals: outer ring (serine taxis) and inner ring (aspartate taxis). | |
To track chemical gradients, E. coli must solve signaling tasks common to all chemotactic behaviors: gradient detection, signal processing, and locomotor control. The chemotaxis machinery of E. coli presents a simple model for exploring the molecular mechanisms of chemosensing and signaling. However, the bizarre physical world in which E. coli lives presents unique difficulties to locomotion and calls for equally unique behavioral solutions. Life in thermokinetic hell The miniscule size of bacteria consigns them to a life that is dominated by viscous drag and Brownian motion (Fig. 2). Yet, despite these daunting physical constraints, E. coli cells swim at speeds of 10-20 body lengths per second. They propel themselves with locomotor organelles called flagella - very different from the flagella of eukaryotic cells - that operate much like the propellers on an ocean liner. |
|
 |
|
| Fig. 2 - Tiny size presents big problems. The small mass of bacterial cells confers little momentum as they swim. If the flagellar motors of an E. coli cell were to stop turning, water viscosity would bring the cell to a full stop after a coasting distance of less than 1 Å. While swimming, collisions with water and solute molecules knock the cells off course. | |
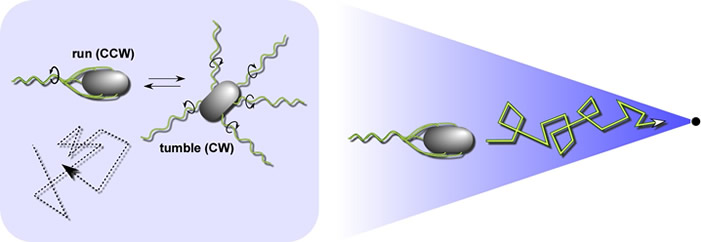
E. coli's optimal foraging strategy In isotropic chemical environments, E. coli swims in a random walk pattern produced by alternating episodes of counter-clockwise (CCW) and clockwise (CW) flagellar rotation (Fig. 3, left panel). In an attractant or repellent gradient, the cells monitor chemoeffector concentration changes as they move about and use that information to modulate the probability of the next tumbling event (Fig. 3, right panel. These locomotor responses extend runs that take the cells in favorable directions (toward attractants and away from repellents), resulting in net movement toward preferred environments. Brownian motion and spontaneous tumbling episodes frequently knock the cells off course, so they must constantly assess their direction of travel with respect to the chemical gradient. |
|
 |
|
| Fig. 3 - Random and biased walks. Left: A random walk in isotropic environments. When the cell's motors rotate CCW, the flagellar filaments form a trailing bundle that pushes the cell forward. When one or more of the flagellar motors reverses to CW rotation, that filament undergoes a shape change (owing to the torque reversal) that disrupts the bundle. Until all motors once again turn in the CCW direction, the filaments act independently to push and pull the cell in a chaotic tumbling motion. Tumbling episodes enable the cell to try new, randomly-determined swimming directions. Right A biased walk In a chemoeffector gradient. Sensory information suppresses tumbling whenever the cell happens to head in a favorable direction. The cells cannot head directly up-gradient because they are frequently knocked off course by Brownian motion. | |
The chemotaxis signaling pathway E. coli senses chemoeffector gradients in temporal fashion by comparing current concentrations to those encountered over the past few seconds of travel. E. coli has four transmembrane chemoreceptors, known as methyl-accepting chemotaxis proteins (MCPs), that have periplasmic ligand binding sites and conserved cytoplasmic signaling domains (Fig. 4). MCPs record the cell's recent chemical past in the form of reversible methylation of specific glutamic acid residues in the cytoplasmic signaling domain (open and filled circles in Fig. 4). Whenever current ligand occupancy state fails to coincide with the methylation record, the MCP initiates a motor control response and a feedback circuit that updates the methylation record to achieve sensory adaptation and cessation of the motor response. A fifth MCP-like protein, Aer, mediates aerotactic responses by monitoring redox changes in the electron transport chain. Aer undergoes sensory adaptation through a poorly-understood, methylation-independent mechanism. The five MCP-family receptors in E. coli utilize a common set of cytoplasmic signaling proteins to control flagellar rotation and sensory adaptation (Fig. 4). CheW and CheA generate receptor signals; CheY and CheZ control motor responses; CheR and CheB regulate MCP methylation state. |
|
| Fig. 4 - Signaling components and circuit logic. E. coli receptors employ a common set of cytoplasmic signaling proteins: CheW and CheA interact with receptor molecules to form stable ternary complexes that generate stimulus signals; CheY transmits those signals to the flagellar motors, CheZ controls their lifetime; CheR (methyltransferase) and CheB (methylesterase) regulate MCP methylation state. Abbreviations: OM (outer membrane); PG (peptidoglycan layer of the cell wall); CM (cytoplasmic membrane). | 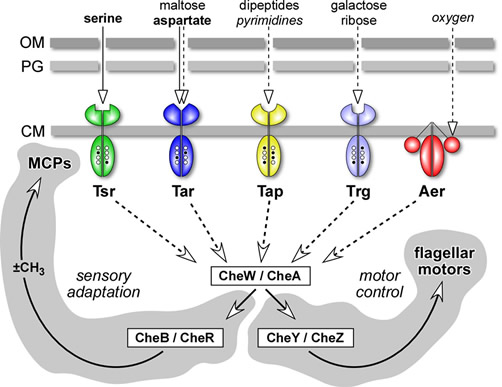 |
As in many biological signaling systems, the signaling currency in the E. coli chemotaxis pathway is reversible protein phosphorylation (Fig. 5). However, the principal signaling chemistry is a bit different in prokaryotes and eukaryotes. CheA is a kinase that uses ATP to autophosphorylate at a specific histidine residue. Phospho-CheA molecules then serve as donors for autokinase reactions that transfer phosphoryl groups to specific aspartate residues in CheY and CheB. Phospho-CheY enhances CW rotation of the flagellar motors; phospho-CheB has high MCP methylesterase activity. The active forms of these response regulators are short-lived because they quickly lose their phosphoryl group through spontaneous self-hydrolysis. CheZ further enhances the dephosphorylation rate of phospho-CheY to ensure rapid locomotor responses to changes in the supply of signaling phosphoryl groups to CheY. CheW couples the autophosphorylation activity of CheA molecules to chemoreceptor control. Receptors, CheW, and CheA form stable ternary signaling complexes that modulate the influx of phosphoryl groups to the CheY and CheB proteins in response to chemoeffector stimuli. |
|
| Fig. 5 - Phosphorelay signaling. The flagellar motors of E. coli spin CCW by default; the signaling pathway modulates the level of phospho-CheY, the signal for CW rotation. Reactions and components that augment CW rotation are depicted in green; those that augment CCW rotation are depicted in red. | 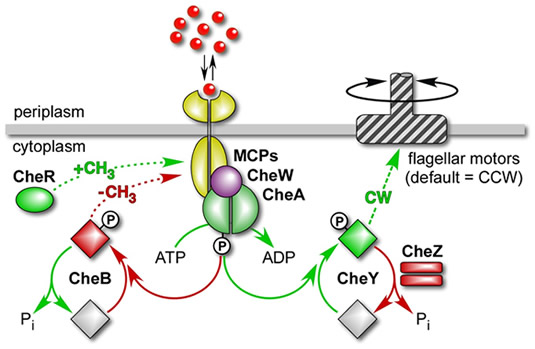 |
Chemoreceptor signaling states The signaling activities of chemoreceptors are described by a two-state model (Fig. 6). Receptor complexes in the CW signaling state activate CheA, producing high levels of phospho-CheY. Receptors in the CCW signaling state deactivate CheA, resulting in low levels of phospho-CheY. Thus, the behavior of the flagellar motors reflects the relative proportion of receptor signaling complexes in the kinase-on and kinase-off conformations. Both chemoeffector binding or release and methylation or demethylation can shift receptor signaling complexes from one state to the other. For example, attractant ligands drive receptors toward the kinase-off state; subsequent addition of methyl groups shifts receptors toward the kinase-on state, reestablishing the steady-state (adapted) balance between the two states and restoring random walk movements. |
|
| Fig. 6 - Two-state model of receptor signaling. The cartoons depict receptor ternary complexes in kinase-active (on) and kinase-inactive (off) signaling states. Changes in chemoeffector occupancy drive the complexes toward one state or the other. During sensory adaptation, changes in receptor methylation level shift signaling complexes toward the opposing state to restore a balance between CCW and CW signal outputs. The actual stoichiometry and structure of receptor signaling complexes are not known. Note that receptor methyl groups are attached to specific glutamic acid (E) residues in the MCP cytoplasmic domain, forming glutamyl methyl esters and neutralizing the negative charge on the glutamyl carboxyl group. Charge neutralization probably plays an important role in controlling receptor output state. | 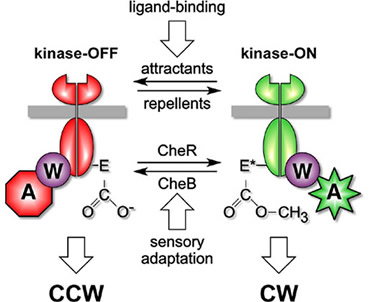 |
