Signaling interactions between bacterial chemoreceptors by Peter Ames, Rebecca Reiser and Sandy Parkinson |
|
| Chemoreceptors of the methyl-accepting chemotaxis protein (MCP) family form clusters, typically at the cell pole(s), in both Bacteria and Archaea. Dennis Bray and colleagues have suggested that clustering might confer network properties that augment the detection sensitivity of receptors (Nature 393:85, 1998). To elucidate the architecture and signaling role of receptor clusters, we investigated interactions between the serine (Tsr) and aspartate (Tar) chemoreceptors of Escherichia coli by constructing Tsr mutations at the six hydrophobic and five polar residues implicated in "trimer of dimers" formation (Fig. 1). | |
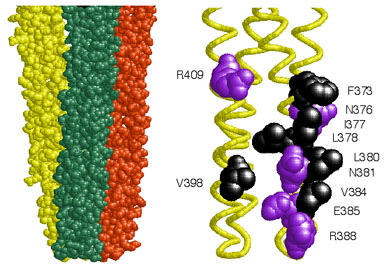
Figure 1. Tsr trimer of dimers. Left: Space-filling view of the cytoplasmic tip of the Tsr signaling domain in the trimer of dimers arrangement. The three dimers converge at their tips, where the contact sites are located. Right: Alpha-carbon backbone of the tip of one dimer, with the trimer contact residues shown in space-filling view. Hydrophobic residues are black, polar residues are purple. |
 |
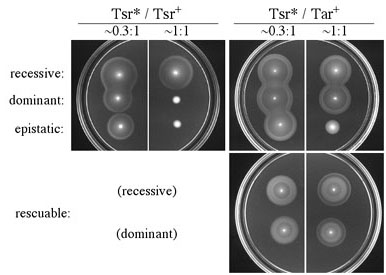
| Tsr mutants with proline replacements could not mediate serine chemotaxis or receptor clustering, properties consistent with a null defect in the alpha-helical trimer contact region. In contrast, alanine and tryptophan mutants, though nonchemotactic, readily formed receptor clusters and exhibited clockwise flagellar rotation, indicative of receptor-coupled activation of the signaling CheA kinase. The receptors with alanine and tryptophan replacements evidently retain the ability to participate in higher-order signaling complexes, but assemble defective complexes that cannot modulate CheA activity in response to serine stimuli. The networking properties of the mutant receptors were examined in complementation tests with wild-type Tsr and Tar. Four complementation patterns were observed (Fig. 2). In addition to the expected recessive and dominant types, some mutants (denoted "epistatic"or "jamming") interfered with Tar function, whereas others (denoted "rescuable") regained the ability to mediate serine chemotaxis in the presence of functional Tar receptors. All tryptophan replacement mutants were jamming; all rescuable mutants had alanine replacements. | |
Figure 2. Complementation behaviors of Tsr trimer contact mutants. Strains carrying a mutant plasmid (Tsr*) and a compatible wild-type plasmid were tested for serine and aspartate chemotaxis on tryptone soft agar plates at different relative expression levels. Epistatic mutations interfered with Tar function, whereas rescuable mutations promoted serine chemotaxis in the presence of wild-type Tar. (Note the outer, serine ring formed at 1:1 co-expression.) |
 |
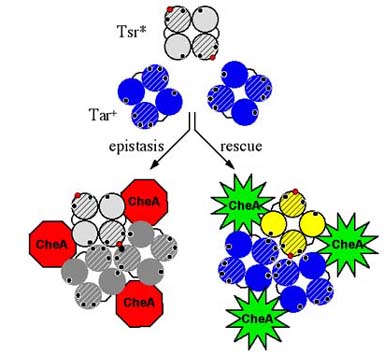
| The unusual complementation phenotypes of Tsr trimer contact mutants suggest that receptor dimers may normally function in higher order signaling teams, possibly trimers of dimers. The epistatic and rescuable behaviors of mutant Tsr dimers could arise through formation of mixed signaling teams with wild-type Tar molecules (Fig. 3). The bulky side chain in tryptophan mutants might prevent stimulus-induced conformational changes in the team, whereas the small side chain in alanine mutants might permit signaling control when teamed with functional receptor molecules. | |
Figure 3. Model of receptor signal teams. Epistasis and rescue of Tsr trimer contact mutants might occur through formation of mixed trimers of dimers. The model depicts a cross-sectional view through receptor dimers. Black circles represent wild-type trimer contacts, red circles represent mutant contact residues. Epistatic defects may distort trimer geometry to prevent modulation of the CheA kinase, whereas rescuable defects may allow functional receptor partners to dictate trimer geometry, thereby restoring stimulus control of CheA signaling activity. |
 |
| Our team model of receptor signaling makes several experimental predictions that have been confirmed in work described in related posters: (1) Tsr and Tar molecules in the same cell can be crosslinked to one another, most likely within their trimer contact regions. (2) Mutations in the trimer contact region of Tar conformationally suppress epistatic mutations in trimer contact residues of Tsr. Taken together, these findings indicate that bacterial chemoreceptor clusters are comprised of signaling teams, seemingly based on trimers of dimers, that can contain different receptor types acting collaboratively. Our current research efforts are focused on elucidating how this network organization confers high-gain signaling characteristics. | |
| Ames, P., C.A. Studdert, R.H. Reiser, and J.S. Parkinson (2002). Collaborative signaling by mixed chemoreceptor teams in Escherichia coli. Proc. Natl. Acad. Sci. USA, 99:7060-7065. | |
