Signaling fragments of a bacterial chemoreceptor by Peter Ames and Sandy Parkinson |
|
| Bacteria employ transmembrane chemoreceptors known as methyl- accepting chemotaxis proteins (MCPs) to mediate a variety of adaptive behaviors. MCP molecules have an external ligand-binding domain for monitoring attractant or repellent levels in the environment and a cytoplasmic signaling domain for communicating with the flagellar rotary motors (Fig. 1). MCPs form ternary complexes with two cytoplasmic proteins, CheA, a histidine kinase, and CheW, which couples CheA activity to chemoreceptor control. To explore the structure and function of the MCP signaling complex, we identified soluble fragments of the serine chemoreceptor, Tsr, that could activate the CheA kinase, both in vivo and in vitro. | |
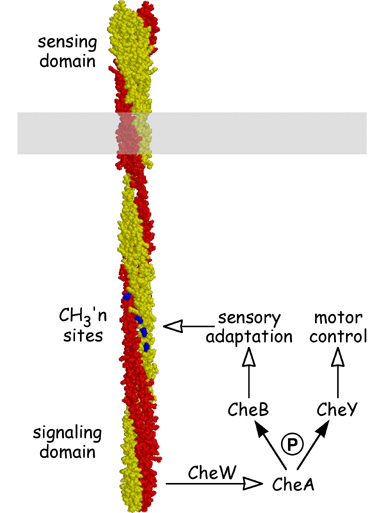
Figure 1. Structure and signaling properties of MCPs. MCP molecules are homodimers, nearly 400 Å in length, of interwound, largely alpha-helical subunits (yellow and red). The MCP signaling domain modulates CheA kinase activity in response to changes in ligand occupancy. CheA in turn passes phosphoryl groups to two effector proteins, CheY, which controls the direction of flagellar rotation, and CheB, which regulates MCP methylation state to bring about sensory adaptation. The methylation sites (blue) are located along the dimer interface and might control signal output by modulating the interactions between MCP subunits. |
 |
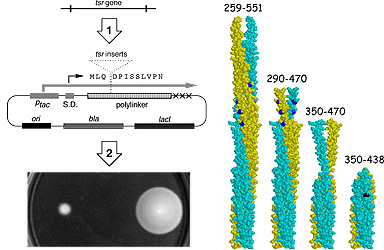
| To identify active signaling fragments of Tsr, we inserted random segments of the tsr coding region into an inducible expression vector and screened for recombinant plasmids that could inhibit or "jam" the chemotactic behavior of a wild-type host strain (Fig. 2, left). We reasoned that Tsr fragments that could bind CheA or CheW would jam chemotactic signaling by competitively titrating these signaling components away from the cell's other chemoreceptors. Moreover, fragments that assemble a functional signaling complex should also interfere with chemotactic behavior by transmitting inappropriate signals to the flagella. | |
| Actively signaling fragments obtained from wild-type Tsr caused excessive clockwise (CW) rotation of the flagellar motors. CW-signaling fragments invariably included the highly conserved cytoplasmic "tip" of the Tsr molecule, indicating that CW signals emanate from that region (Fig. 2, right). Some CW fragments lacked the methylation sites involved in sensory adaptation, demonstrating that MCP methylation is not essential for CW signaling. However, longer fragments containing the methylation sites only caused CW rotation if methylated, implying that the unmethylated form of MCP molecules actively interferes with assembly or function of the CW signaling complex. | |
Figure 2. Tsr signaling fragments. (Left) Scheme for identifying active fragments. (1) Random pieces of the tsr coding region were cloned into an inducible expression vector. (2) Recombinant plasmids were screened in a wild-type host for ability to jam chemotaxis upon induction. (Right) Fragments that jam chemotaxis and enhance CW flagellar rotation. Methylation sites (blue) are not essential for these activities. The shortest active fragment (350-438) requires a point mutation (black) to stabilize its CW-signaling conformation. |
 |
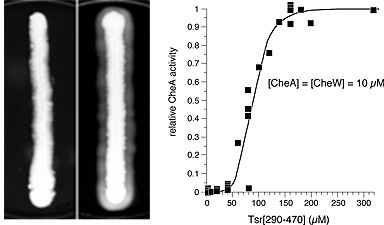
Cells that lack MCPs cannot generate CW signals and, consequently, rotate their flagella exclusively in the counter-clockwise direction. Tsr fragments with CW signaling activity cause such cells to spread faster through soft agar, a behavior we call "pseudotaxis" (Fig. 3, left). Using the pseudotaxis effect to monitor CW signaling activity, we progressively trimmed our shortest Tsr fragment to determine the minimum size of the CW signaling segment. The current record holder is an 89-residue fragment centered on the signaling tip (Fig. 2). Several larger CW fragments were purified and shown in vitro to activate the CheA kinase. CW fragments acted in a highly cooperative manner, with perhaps 8-10 fragments needed to activate a single CheA molecule (Fig. 3, right). Activation also required the CheW coupling protein, suggesting that Tsr fragments assemble ternary signaling complexes comparable to those made by full-length MCP molecules in vivo. |
|
Figure 3. Assays for CW signaling. (Left) Induction of pseudotactic spreading by a Tsr fragment in vivo. The host cells lack other MCPs and spread faster through soft agar upon expression of a fragment that enhances CW flagellar rotation. (Right) Stimulation of CheA activity by a CW-signaling Tsr fragment in vitro. Fragment action is CheW- dependent and highly cooperative. |
 |
| Our studies of Tsr fragments have provided important new insights into the signaling mechanisms of bacterial chemoreceptors. For example, it now appears that several MCP molecules must cooperate in the assembly and function of ternary signaling complexes. In addition, the in vivo and in vitro assays that we developed for Tsr signaling fragments have enabled us to identify the binding contacts and other determinants of chemoreceptor function, as described in other posters. | |
| Ames, P. & J.S. Parkinson (1994) Constitutively signaling fragments of Tsr, the E. coli serine chemoreceptor. J. Bacteriol. 176: 6340-6348. | |
| Ames, P., Y.A. Yu & J.S. Parkinson (1996) Methylation segments are not required for chemotactic signaling by cytoplasmic fragments of Tsr, the methyl-accepting serine chemoreceptor of Escherichia coli. Mol. Microbiol., 19: 737-746. | |
