Signaling control surfaces in the CheA kinase by Jinshi Zhao and Sandy Parkinson |
|
| The histidine autokinase CheA is the central processing unit of bacterial chemotaxis. CheA forms stable signaling complexes with transmembrane chemoreceptors and the small CheW protein, which couples CheA autophosphorylation to receptor control. In the absence of attractant stimuli, the receptors activate CheA, roughly 200-fold over its uncoupled activity. Upon detecting an attractant increase, the chemoreceptor signaling complexes shift to a conformation that deactivates CheA, thereby slowing the flux of phosphoryl groups to the CheY and CheB proteins to trigger appropriate locomotor responses and subsequent sensory adaptation, respectively. | |
Figure 1. CheA domain architecture. CheA functions as a homodimer; each subunit contains five distinct domains (P1-P5) Autophosphorylation is a trans reaction between the ATP-binding domain (P4) in one subunit and the phosphorylation site domain (P1) in the other. Most of the dimerization determinants lie in the P3 domain. The P5 domain contains determinants for CheW-binding and chemoreceptor coupling control of CheA autophosphorylation activity. In this study, these determinants were investigated with missense mutations and by chemical modification of cysteine reporter residues. |
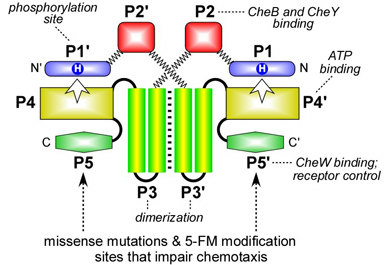 |
| This study explored the C-terminal P5 domain of CheA (Fig. 1), which binds the CheW protein and couples CheA autophosphorylation to receptor control. To identify amino acid residues and other P5 structural features critical for CheW-binding and receptor coupling control, we first isolated and characterized P5 missense mutations that impaired chemotactic ability. Some P5 missense mutations caused defects in CheW-binding and receptor coupling control (Fig. 2, black residues). Other P5 lesions (Fig. 2, blue residues) had no effect on receptor-mediated activation, but prevented the signalng complex from entering the deactivated state in response to an attractant stimulus. Another group of P5 mutations (Fig. 2, red residues) caused chemotaxis defects in vivo, but no apparent biochemical defects in vitro. These mutants could represent previously unsuspected P5 activities for which there is no in vitro assay. Finally, some mutations at buried hydrophobic residues (Fig. 2, white residues) disrupted P5 folding and stability. | |
Figure 2. Summary of P5 missense mutations. Structure of the P3-P5 domains of E.coli CheA modeled from the atomic coordinates of Thermotoga maritima CheA. The P5 domains (green; one backbone, one space-filled) indicate the residue positions of missense mutations that cause different functional defects (white, black, red, blue). |
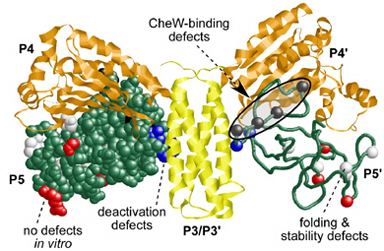 |
| The P5 missense mutations not only identified a putative CheW-binding surface (see Fig. 2), but also suggested that dynamic motions of the P5 domain could play important roles in the assembly and operation of receptor signaling complexes. To explore these ideas further, we carried out a cysteine-scanning analysis of the CheA-P5 domain. We first constructed a CheA protein with serines in place of the three native cysteine residues. The cysteine-less CheA had wild-type function both in vivo and in vitro. Then, we introduced a single cysteine residue at various P5 surface positions and examined the in vitro activities of the CheA-P5/Cys proteins before and after modification with cysteine-targeted 5-fluorescein maleimide. | |
| We found that attaching a bulky fluorescein group to cysteine residues in or near the putative CheW-binding surface disrupted CheW-binding and receptor coupling. However, modifications at some distal sites also impaired CheW-binding or receptor-mediated activation or deactivation. Many of those distal sites fell in flexible loops between P5 secondary structure elements, which is consistent with the idea that 5-FM modification at those sites influenced the dynamic motions and/or conformational stability of the P5 domain, which in turn affected CheW-binding and receptor coupling control. | |
Figure 3. P5 and CheW signaling surfaces. The P5 and CheW structures are closely related. Portions shown in black contain the critical binding determinants. Our cysteine-scanning analysis also identified a P5 surface distinct from the CheW-binding region that was important for receptor control of CheA. |
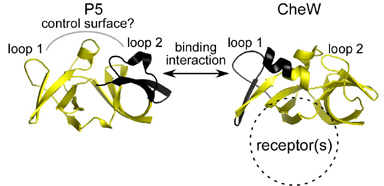 |
| Our current view of the CheA-CheW interaction is summarized in Fig. 3. The CheA-CheW binding interaction occurs between determinants in loop 2 of P5 subdomain 1 and loop 1 of CheW subdomain 2. Conformational communication between subdomains within and between P5 and CheW is most likely an important factor in ternary complex function. Chemoreceptors appear to bind to the subdomain interface in CheW and may transmit stimulus-induced conformational changes to the CheA molecule through the CheW-P5 binding contacts. | |
| The evidently dynamic natures of P5 and CheW have probably hindered high-resolution structural studies of ternary signaling complexes, but with the new insights provided by these P5 studies, it may now be possible to devise mutationally altered ternary complex components with more stable structures that can shed additional light on the mechanism of CheA activation and deactivation by receptors and CheW. | |
| Zhao, J. and J.S. Parkinson (2006) Mutational analysis of the chemoreceptor-coupling domain of the Escherichia coli chemotaxis signaling kinase CheA. J. Bacteriol. 188:3299-3307. | |
| Zhao, J. and J.S. Parkinson (2006) Cysteine-scanning analysis of the chemoreceptor-coupling domain of the Escherichia coli chemotaxis signaling kinase CheA. J. Bacteriol. 188:4321-4330. | |
