Physical interactions between bacterial chemoreceptors by Claudia Studdert and Sandy Parkinson |
|
| Bacterial chemoreceptors of the methyl-accepting chemotaxis protein (MCP) family form supramolecular clusters that operate as primitive neural networks. As described in a related poster, we found that proline replacement mutations at the trimer contact sites in Tsr, the serine chemoreceptor, prevented cluster formation. In contrast, tryptophan replacements at the contact sites allowed Tsr to form defective clusters in which signaling by wild-type Tar, the aspartate receptor, was blocked. These findings indicate that bacterial chemoreceptors may signal collaboratively in teams (i.e., trimers of dimers) that can contain receptors of different types. The team signaling model predicts that Tsr and Tar molecules in the same team should be close enough to join with a bifunctional crosslinking agent. We tested this idea by using an affinity handle to purify Tar (and any molecules attached to it) after treating cells with a chemical crosslinker (Fig. 1). | |
Figure 1. Experimental scheme for in vivo crosslinking of chemoreceptors. Wild-type or mutant Tsr receptors were coexpressed with functionally normal Tar molecules bearing a 6X-His affinity tag (asterisk). After forming receptor clusters, the host cells were treated with crosslinking agent, then disrupted to collect membranes. Proteins in the inner membrane fraction were solubilized in detergent and passed across an affinity resin to purify those carrying 6X-His tags. After cleaving crosslinks, receptor subunits were identified by gel electrophoresis and immunoblotting. |
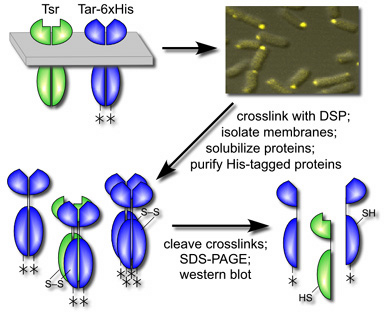 |
| Treatment of cells with a membrane-permeant crosslinking agent caused wild-type Tsr subunits to co-purify with the tagged Tar molecules (data not shown). Crosslinking of Tsr to Tar was not dependent on the cytoplasmic CheW and CheA proteins, which are also components of receptor signaling complexes, nor on the CheB and CheR proteins, the enzymes that interact with MCP molecules to add and remove methyl groups. Thus, Tsr appears to make a direct, unassisted contact to Tar. That interaction most likely occurs through their trimer contact regions, because a proline replacement at a Tsr contact site prevented crosslinking to Tar, whereas a tryptophan replacement at the same site did not. These findings define several organizational stages in the formation of receptor signaling teams and clusters (Fig. 2). | |
Figure 2. Model of chemoreceptor signal team formation and organization. After insertion into the cytoplasmic membrane and dimerization, receptor molecules coalesce into "squads" that can contain receptors of different types. Squads form in the absence of the cytoplasmic CheA and CheW proteins and most likely correspond to trimers of dimers. Receptor squads subsequently recruit the CheA and CheW proteins to build signaling teams that activate and inhibit CheA kinase activity in response to chemical stimuli detected by the periplasmic ligand-binding domains of the chemoreceptors. Different signaling teams may share CheA and CheW partners, leading to formation of macroscopic chemoreceptor clusters. |
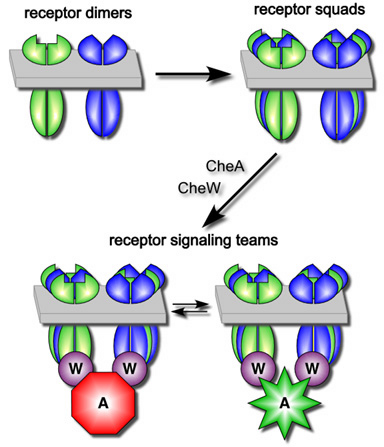 |
| We propose that receptor dimers associate in "squads" through their trimer of dimers contacts. Because the trimer contact residues are identical in all five E. coli members of the MCP family, different receptors can join the same trimer/squad, which accounts for our crosslinking signal. Receptor squads bind CheW and CheA to form signal teams, which might share binding connections with other teams to form macroscopic receptor clusters. If the receptor molecules in a team cooperate to control a shared CheA molecule, this would provide a substantial signal amplification factor. To explore this possibility, we are devising in vitro systems to study the composition, architecture and behavior of receptor signaling teams. | |
| Ames, P., C.A. Studdert, R.H. Reiser, and J.S. Parkinson (2002). Collaborative signaling by mixed chemoreceptor teams in Escherichia coli. Proc. Natl. Acad. Sci. USA, 99:7060-7065. | |
