Methylation-independent aerotaxis: How does Aer tell time? by Sergei Bibikov, Drew Miller, Khoosheh Gosink and Sandy Parkinson |
|
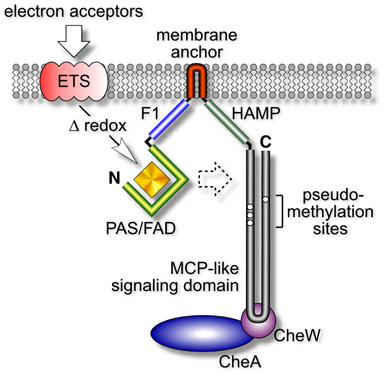
| Aer is a membrane-associated protein that mediates aerotactic responses in E. coli (Fig. 1). It has an N-terminal PAS domain that binds flavin adenine dinucleotide (FAD), which it uses to monitor oxygen-related cellular redox changes. Those sensory inputs are in turn transmitted, by an uncharted route, to the C-terminal half of Aer, which closely resembles the output signaling domains of chemoreceptors known as methyl-accepting chemotaxis proteins (MCPs). Methylation endows MCPs with the abilty to detect chemical changes over time (see below). However, the "methylation sites" in the Aer signaling domain do not conform to the consensus MCP motif, suggesting that Aer might be a methylation-independent transducer. If so, how could it detect temporal changes in cellular redox conditions? | |
Figure 1. Domain organization and signaling activities of Aer. Aer monitors the cell's electron transport activity through a redox-sensitive FAD cofactor. Those signals are communicated, either directly or indirectly, to the Aer signaling domain, which generates flagella-controlling signals through ternary complexes with the CheA and CheW proteins. The Aer signaling domain resembles the highly conserved signaling domain of methyl-accepting chemotaxis proteins, except for unorthodox methylation site residues. Aer functions as a dimer, but the functional and structural interactions between the two subunits have not yet been elucidated. |
 |
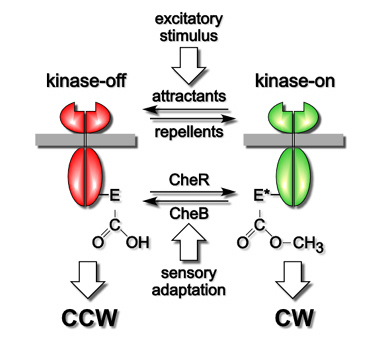
| MCPs use their methylation state as a memory store for making temporal comparisons of attractant and repellent levels. Whenever current conditions, reflected in the fraction of ligand-occupied receptors, differ from those over the past few seconds, represented by the average methylation state of the molecules, MCPs trigger a motility response and begin updating their methylation state to correspond to the new chemical environment. The timing mechanism of MCP molecules is summarized in Figure 2. | |
| MCPs have two signaling states, one that activates the CheA kinase to enhance clockwise flagellar rotation and one that inhibits CheA to enhance counter-clockwise rotation. Attractant and repellent ligands drive the cell's population of MCP molecules toward one signaling state to elicit motor responses. Subsequent changes in MCP methylation drive MCPs toward the other signaling state to bring about sensory adaptation. The opposing activities of two specific enzymes, CheR, a methyltransferase, and CheB, a methylesterase, control MCP methylation state. CheR converts specific glutamic acid residues in the MCP signaling domain to glutamyl methyl esters, using S-adenosyl methionine as the methyl donor. CheB hydrolyzes those methylated residues, releasing methanol and restoring glutamic acid. CheR activity is unregulated, whereas CheB is feedback-regulated by MCP output signals. Thus, the temporal disparity between rapid motor response and slower feedback adaptation provides the cell with a few second memory of its chemical past. | |
Figure 2. Two-state model of MCP signaling and time-keeping.
|
 |
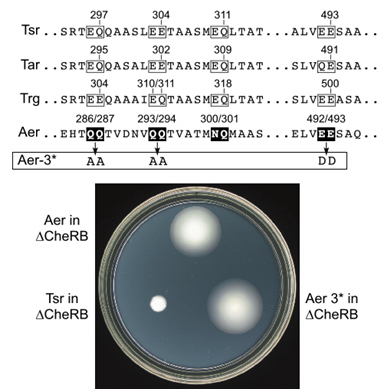
| To determine whether Aer-mediated aerotaxis is a methylation-independent behavior, we examined Aer for CheR- or CheB-dependent covalent modifications, assessed Aer function in cheR and cheB mutants, and examined the behavior of Aer "methylation site" mutants. These experiments demonstrated that Aer function was not dependent on CheR or CheB, nor on methylatable sites in the signaling domain, nor on any of the conventional MCPs in E. coli. For example, a strain lacking all four of the E. coli MCPs as well as CheR and CheB mediated robust aerotactic responses when supplied with a wild-type Aer gene (Fig. 3). Conventional chemoreceptors such as Tsr, the serine receptor, failed to function in the same strain background. Moreover, amino acid replacements at the putative "methylation sites" in Aer, chosen to preclude any possibility of methylation or demethylation, had no deleterious effect on Aer function (Fig. 3). | |
Figure 3. Methylation site alterations in Aer and their lack of functional consequences. The sites of methylation in conventional MCPs are indicated by their residue numbers. The corresponding residues in wild-type Aer were converted to residues that are known not to serve as substrates for CheR or CheB modifications. The resulting Aer-3* mutant still mediated aerotaxis in a host strain lacking all MCPs and CheR and CheB. |
 |
| Our findings imply that sensory adaptation occurs by a methylation-independent mechanism in the Aer signaling pathway. So how does Aer tell time? Maybe it doesn't have to; perhaps the cellular redox changes that trigger Aer signals are themselves transient. We hope to test this possibility by reconstructing the Aer signaling pathway in a cell-free system. | |
| Bibikov, S.I., Miller, A.C., Gosink, K. and J.S. Parkinson (2004) Methylation-independent aerotaxis mediated by the Escherichia coli Aer protein. J. Bacteriol. 186:3730-3737. | |
