Crosslinking snapshots of chemoreceptor squads by Claudia Studdert and Sandy Parkinson |
|
| The team signaling model for bacterial chemoreceptors (see related posters) proposes that receptor dimers of different detection specificities form mixed trimers of dimers (receptor "squads"), which then recruit the cytoplasmic proteins CheA and CheW to form signaling teams, typically clustered at the poles of the cell. To test key predictions of this model, we devised in vivo crosslinking methods to ask if mixed receptor squads could form in the absence of CheA and CheW and, if so, whether their underlying structural interactions conformed to trimer-of-dimers geometry. | |
| Our general approach was to introduce single cysteine residues into two different receptors and to analyze their crosslinking products when intact cells were subjected to sulfhydryl-targeted crosslinking conditions. We looked for reporter sites predicted to crosslink in the trimer-of-dimers structure and at which a cysteine replacement could be tolerated with no loss of receptor function. The cysteine-bearing receptors were expressed from compatible plasmids with independently regulatable expression controls. The host cells contained no other receptors nor any soluble chemotaxis proteins known to interact with receptor signaling domains. | |
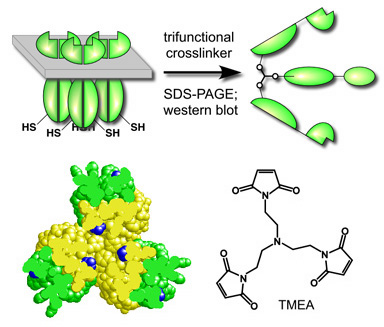
| One crosslinking scheme used a trifunctional crosslinking reagent (TMEA) to exploit the unique trigonal symmetry of the trimer of dimers (Fig. 1). As reporter sites, we selected residue 366 in Tsr, the serine receptor, and corresponding positions in the aspartate receptor (Tar-364) and the ribose-galactose receptor (Trg-374). (All three cysteine replacement mutants retained full receptor function.) In the axial subunits of the trimer of dimers, the side chains at these positions are solvent-exposed and spaced appropriately to react with the maleimide groups in TMEA (Fig. 1). | |
Figure 1. An in vivo crosslinking scheme for capturing receptor subunits from trimers of dimers. Receptors bearing cysteine reporters were expressed at physiological levels in cells lacking CheA and CheW. The cells were treated with TMEA, a trifunctional crosslinking reagent, and the crosslinked receptor molecules were analyzed by denaturing gel electrophoresis and antibody detection. The space-filled structure shows a cross-section through the trimer of dimers, viewed toward the cytoplasmic tip. The cysteine reporters (blue) lie just above the trimer contact region and in the axial subunits (yellow) of each dimer are spaced about 11 Å apart. TMEA (not to the same scale) has three SH-reactive maleimide groups spaced 10.3 Å apart. |
 |
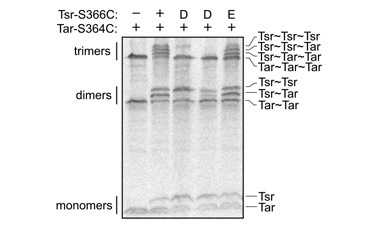
| Upon TMEA treatment, cysteine-bearing receptors formed two- and three-subunit crosslinking products with high efficiency (Fig. 2). Co-expression of different receptors produced mixed crosslinking products (e.g., Tar~Tsr, Tsr~Trg) whose proportions reflected the relative expression levels of the various receptor subunits. Trimer contact site mutations that impair cluster formation prevented formation of mixed crosslinking products. In contrast, receptors with jamming trimer contact lesions, which can cluster and spoil the function of other receptors, readily formed mixed crosslinking products. | |
Figure 2. Crosslinking of cysteine-bearing Tsr and Tar molecules. Lane 1: Tar-S364C alone formed two- and three-subunit crosslinks. Lane 2: Tar-S364C plus Tsr-S366C formed a variety of mixed crosslinking products. Lanes 3 & 4: Nonclustering mutations (D: I377P, E385P) prevented Tsr-S366C from forming 3-subunit crosslinks and mixed two-subunit crosslinks. Lane 5: A clustering-proficient, jamming mutation (E: N381W) did not affect the crosslinking pattern of Tsr-S366C. |
 |
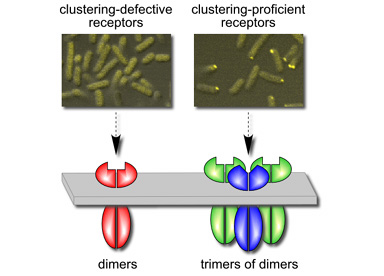
| The clustering, signaling, and crosslinking behaviors of mutant receptor molecules indicate that trimers of dimers are both structural and functional precursors of chemoreceptor signaling teams (Fig. 3). | |
Figure 3. Relationship between trimer-of-dimers formation and receptor clustering. Mutations at trimer contact sites that disrupt cluster formation also block trimer-of-dimers formation. Clustering-proficient receptors (wild-type and jamming mutants) form trimers of dimers whose compositions are determined by the relative proportions of different receptor types in the dimer pool. |
 |
| Our crosslinking results lend strong support to the team model of receptor signaling. These new methods should enable us to elucidate the functional architecture of chemoreceptor teams and ultimately to understand the source of the prodigious signal amplification produced by the bacterial chemotaxis machinery. | |
| Studdert, C.A. and J.S. Parkinson (2004) Crosslinking snapshots of bacterial chemoreceptor squads. Proc. Natl. Acad. Sci. USA 101:2117-2122. | |
