Chemotactic signaling without a target acquisition domain by Knut Jahreis, Tom Morrison, Andrés Garzón and Sandy Parkinson |
|
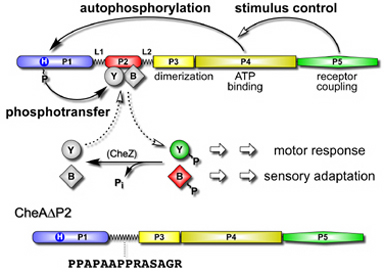
| CheA, a cytoplasmic kinase controlled by transmembrane receptors, is the central signal-processing unit in bacterial chemotaxis. CheA autophosphorylates, using ATP, then donates its phosphoryl groups to CheB and CheY, the response regulator proteins that control flagellar rotation and sensory adaptation. These phosphotransfer reactions are quite specific: CheA is not a phosphodonor to the many other response regulator proteins in the cell that use the same signaling chemistry as CheB and CheY. CheA has a modular architecture, with different signaling functions allocated to different domains. The P2 domain, which binds CheY and CheB with high affinity, might confer CheA's signaling specificity. To test this idea, we constructed a CheA molecule in which the P2 domain had been replaced by a flexible linker (Fig. 1). | |
Figure 1. Domain organization and signaling activities of CheA. CheA regulates flagellar movements and stimulus sensitivity by activating the effector proteins CheB and CheY through phosphorylation. The P2 domain of CheA binds both target proteins to enhance phosphotransfer rates. To determine whether P2 confers specificity to CheA signaling, we extirpated the P2 domain from CheA and replaced it with a flexible linker. |
 |
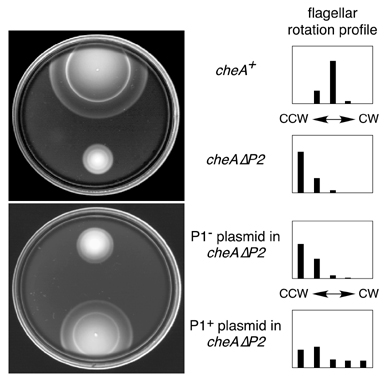
| The CheAΔP2 protein had essentially wild-type autophosphorylation activity, which responded normally to chemoreceptor control. However, the mutant protein exhibited a reduced rate of phosphotransfer to both CheB and CheY. Moreover, cells containing CheAΔP2 instead of CheA migrated slowly on chemotaxis test plates and had low rates of clockwise flagellar rotation, consistent with inefficient phosphotransfer to CheB and CheY (Fig. 2). Nevertheless, the mutant colonies evinced residual chemotactic ability, which improved dramatically when we expressed high levels of the CheA phosphorylation site (P1) domain in the cells. Free P1 domains also restored a more normal pattern of flagellar rotation to the cells (Fig. 2). | |
Figure 2. Chemotaxis and flagellar rotation phenotypes of CheAΔP2. Left: Chemotaxis assays on tryptone soft agar plates. As the colonies grow, the cells establish attractant gradients through metabolism. Chemotactic strains follow the gradients, forming "rings" of concentrated cells at the attractant fronts. The CheAΔP2 mutant migrates much slower than wild-type, but still forms chemotactic rings. Chemotaxis improved dramatically upon high level expression of free phosphorylation site (P1) domains in the cells. Right: Rotation patterns of cells tethered (with antibody molecules) to microscope slides by a single flagellum. The majority of wild-type cells reversed directions of rotation frequently during the observation period, whereas most of the CheAΔP2 individuals rotated exclusively in the counter-clockwise direction. The P1-repaired CheAΔP2 cells have a less CCW-biased flagellar rotation pattern. |
 |
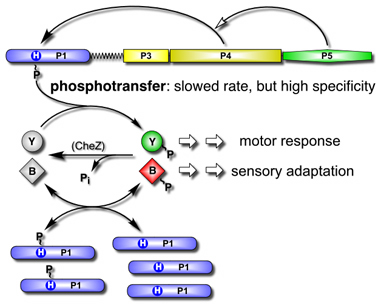
| The ability of free P1 domains to cure the CheAΔP2 mutant implies that the mutant protein, despite slowed phosphotransfer rates, retains sufficient signaling specificity for chemotactic responses. Perhaps phosphotransfer specificity resides in P1 and the free P1 domains enhance behavior by buffering the cellular pool of CheAΔP2-generated phosphoryl groups (Fig. 3). Thus, although the P2 domain normally augments the phosphotransfer process through target acquisition, it does not play a critical role in CheA signaling specificity. We conclude that the main signaling specificity determinants of CheA that prevent excessive crosstalk with heterologous response regulators reside in the P1 phosphorylation site domain. | |
Figure 3. Model of chemotactic signaling by P1-assisted CheAΔP2. CheAΔP2 retains high specificity for phosphotransfer to CheB and CheY, but the transfer rates are reduced. According to the model, free P1 domains create a reservoir of signaling phosphates by acquiring phosphoryl groups from phospho-CheB and phospho-CheY through exchange reactions. In effect, P1 creates a pool of phosphoryl groups, buffered against loss through hydrolytic dephosphorylation, that are available for exchange with CheY to augment clockwise motor rotation. |
 |
| These findings demonstrate that the P2 domain is not an essential module for CheA signaling activities. Rather, it seems to represent an evolutionary embellishment that improved phosphotransfer rates, but not their specificity. | |
| Jahreis, K., Morrison, T.B., Garzon, A. and J.S. Parkinson (2004) Chemotactic signaling by an Escherichia coli CheA mutant that lacks the binding domain for phosphoacceptor partners. J. Bacteriol. 186:2664-2672. | |
